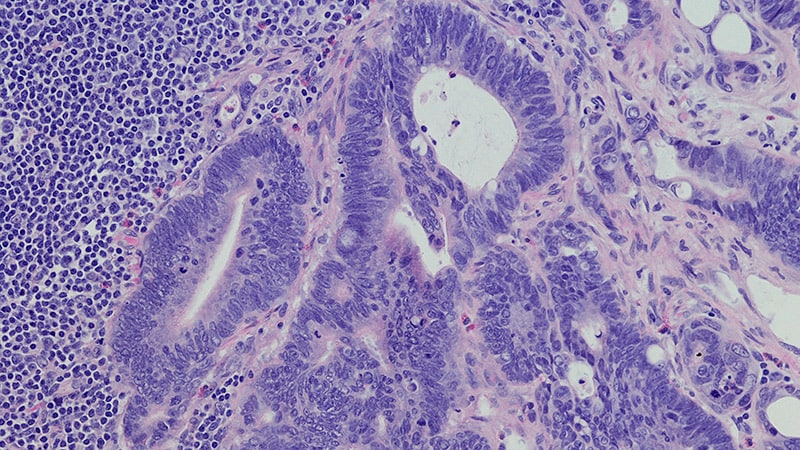
The US Food and Drug Administration (FDA) has approved sotorasib (Lumakras, Amgen Inc.) with panitumumab (Vectibix, Amgen Inc.) for the treatment of certain adult patients with metastatic colorectal cancer (mCRC). Specifically, the combination therapy is indicated for those with KRAS G12C-mutated mCRC, as determined using an FDA-approved test…
FDA Approves Sotorasib + Panitumumab for mCRC
Leave a Comment Leave a Comment



